General Overview
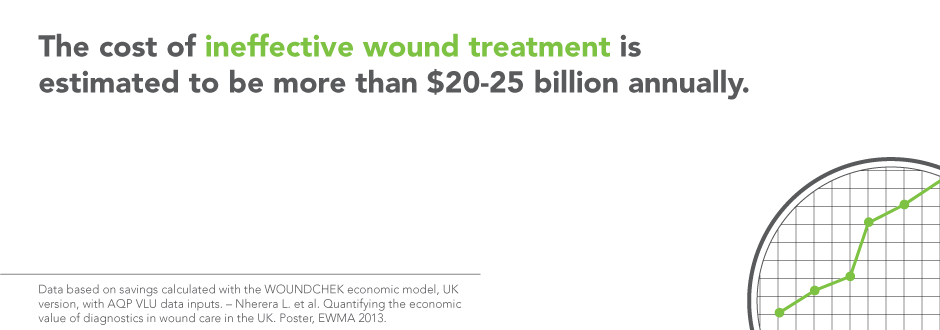
WOUNDCHEK™ Laboratories is focused on the development of novel wound diagnostic products to help improve outcomes in wound care. Currently, the cost of ineffective treatment is estimated to be more than $20-25 billion¹Data based on savings calculated with the WOUNDCHEK economic model, UK version, with AQP VLU data inputs. – Nherera L. et al. Quantifying the economic value of diagnostics in wound care in the UK. Poster, EWMA 2013. annually. Clinical experts agree the use of point of care wound diagnostic devices could reduce the cost of ineffective treatment and delayed healing by being able to target the right therapy, on the right patient, at the right time, and for the right length of time²World Union of Wound Healing Societies (WUWHS). Principles of best practice: Diagnostics and wounds. A consensus document. London: MEP Ltd, 2008.. A resulting financial benefit to health care systems can be achieved through significant reduction of the expenses associated with poor targeting of treatment, delayed healing, and avoidable complications. WOUNDCHEK™ Laboratories launched the world’s first and only point of care test to detect EPA (elevated protease activity) in chronic wounds in 2012, and over the past several years has become a recognized leader in wound diagnostics.
Mission
The company’s vision is to provide clinicians with better, accurate, timely, actionable, and easy to access information, leading to improved outcomes through point of care wound diagnostics. WOUNDCHEK™ Laboratories aims to be the leading and dominant enterprise in wound diagnostics by providing medical professionals with the diagnostic information to:
-
Identify wounds which are not healing
-
Intercept issues and early warnings that cause poor wound healing or initiate wounds
-
Aid in determination of best therapeutic routes
-
Monitor the effectiveness of a course of treatment
Activities
Based in the UK and the USA, WOUNDCHEK™ Laboratories' staff are focused on product development, clinical research, and working with its distribution partners to develop the emerging wound diagnostics market.
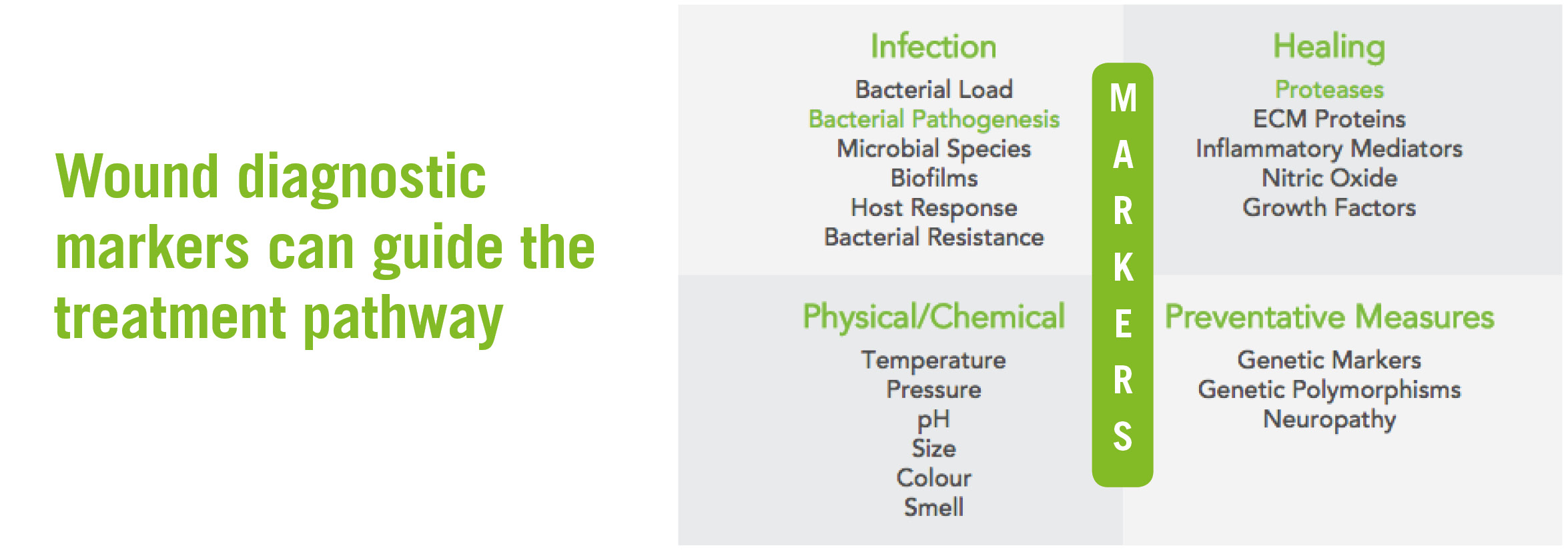
Our research is centred on two critical questions, identified by clinical experts as primary pieces of diagnostic information needed to direct treatment pathways in the majority of chronic wounds:
-
Does the wound have chronic inflammation?
-
Is there an active bacterial pathogenesis?
ISO 13485:2016 certified quality management system.
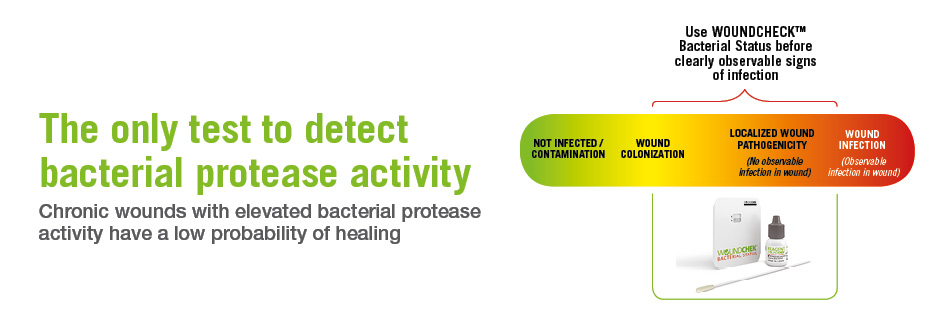
Bacterial Virulence
Bacterial proteases have been described as the most important class of virulence factors and play a key role in the establishment of wound infections, ultimately impeding the healing response and keeping the wound in a chronic state1Koziel J, Potempa J. Protease-armed bacteria in the skin. Cell Tissue Res. 2013; 351(2): 325-37.. They are produced by both Gram-negative and Gram-positive bacteria associated with chronic wound colonization2Wysocki AB, Bhalla-Regev SK, Tierno PM, Steven-Riley M, Wiygul R. Proteolytic Activity by Multiple Bacterial Species Isolated from Chronic Venous Leg Ulcers Degrades Matrix Substrates. Biol Res Nurs. 2012; 15(4): 407-415..
The detection of bacterial protease activity in a chronic wound would be indicative of the presence of bacterial virulence which is a precursor to clinical signs and symptoms of infection3Thomson PD, Smith DJ. What is infection? Am. J. Surg. 1994; 167(No. 1A): 7S-10S.,4Dow G, Browne A, Sibbald RG. Infection in chronic wounds: Controversies in diagnosis and treatment. Ostomy Wound Management 1999; 45 (8): 23-40. .
Bacterial virulence is undesirable since, at this stage, the wound is in a part of the wound infection continuum that typically requires intervention.
WOUNDCHEK™ Bacterial Status — Now available in countries accepting CE mark or FDA clearance for use in clinics authorized to conduct 'Moderately Complex' testing.
|
PRODUCT CODE |
DESCRIPTION |
CONTAINS |
|
360006 |
WOUNDCHEK™ Bacterial Status |
6 tests (all material needed) |
|
360010 |
WOUNDCHEK™ Bacterial Status |
3 positive & 3 |
Evidence
Posters & Articles
![]() Baines, D. et al. 2022. Effectiveness of Testing Hard-to-Heal Wounds for Bacterial Protease Activity: A Randomised Clinical Trial
Baines, D. et al. 2022. Effectiveness of Testing Hard-to-Heal Wounds for Bacterial Protease Activity: A Randomised Clinical Trial
![]() Serena, T. et al. 2022. Bacterial protease activity: a prognostic biomarker of early wound infection
Serena, T. et al. 2022. Bacterial protease activity: a prognostic biomarker of early wound infection
![]() Serena, T. et al. 2021. Bacterial protease activity as a biomarker to assess the risk of non-healing in chronic wounds: Results from a multicenter prospective cohort clinical trial
Serena, T. et al. 2021. Bacterial protease activity as a biomarker to assess the risk of non-healing in chronic wounds: Results from a multicenter prospective cohort clinical trial
![]() Serena, T. et al. 2017. A Rapid Point of Care Test for Bacterial Protease in Chronic Wounds is Predictive of Amputation Risk
Serena, T. et al. 2017. A Rapid Point of Care Test for Bacterial Protease in Chronic Wounds is Predictive of Amputation Risk
![]() Serena, T. et al. 2015. Bacterial proteases: A marker for a 'state of pathogenesis' in chronic wounds
Serena, T. et al. 2015. Bacterial proteases: A marker for a 'state of pathogenesis' in chronic wounds
![]() Serena, T. et al. 2015. Bacterial protease activity in chronic wound fluid, a potential indicator of pathogenicity even in the absence of overt signs of infection.
Serena, T. et al. 2015. Bacterial protease activity in chronic wound fluid, a potential indicator of pathogenicity even in the absence of overt signs of infection.
![]() Benson, R. et al. 2013. Bacterial protease activity, an indicator of bacterial pathogenicity in chronic wounds even in the absence of overt clinical signs.
Benson, R. et al. 2013. Bacterial protease activity, an indicator of bacterial pathogenicity in chronic wounds even in the absence of overt clinical signs.
For older Posters & Articles send a request to info@wounchek.com
Consensus Documents
![]() Armstrong, D. et al. 2020. Principles of Best Diagnostic Practice in Tissue Repair and Wound Healing: An Expert Consensus
Armstrong, D. et al. 2020. Principles of Best Diagnostic Practice in Tissue Repair and Wound Healing: An Expert Consensus
![]() International Wound Infection Institute, 2016. Wound Infection in Clinical Practice
International Wound Infection Institute, 2016. Wound Infection in Clinical Practice
![]() Wounds International, Serena T., et al, 2016. BPA Made Easy
Wounds International, Serena T., et al, 2016. BPA Made Easy
![]() Wounds International 2015, Vol 6 Issue 4. The Use of a point-of-care test for bacterial protease activity in chronic wounds
Wounds International 2015, Vol 6 Issue 4. The Use of a point-of-care test for bacterial protease activity in chronic wounds
For older Consensus Documents send a request to info@wounchek.com
Symposia
Baines, D. et al. 2020. Experiences of testing wounds for bacterial protease activity in a community setting.
Wilkens, J. EWMA 2020. Introduction of Woundchek™ Labs.
Jack Wilkens
Chief Executive Officer
Former Systagenix Vice President of Diagnostics Development, CEO of RevDia, which specializes in infectious disease point of care diagnostic testing. Prior to RevDia, he was Chief Operating Officer for Inverness Medical Innovations (now Alere - ALR-NYSE), a world leader in point of care rapid diagnostics. He has also held senior management positions with Unipath, IMI, Sterling Diagnostics / AGFA and Polaroid Corporation. Mr. Wilkens has a BS in Chemical Engineering and an MBA.

Simon Bayliff
Vice President, Research & Development
Simon is a Biochemistry & Physiology graduate with over 35 years' experience developing and commercializing products in the Medical Device and In-Vitro Diagnostic fields. He has worked in 'start-up' environments as well as for large companies including Abbott Laboratories and Johnson & Johnson.

Malcolm Williams
Chief Financial Officer
Malcolm is a fellow of the chartered institute of management accountants (UK) as well as having an honours degree in accountancy and finance. He spent 22 years with Johnson and Johnson working mainly in medical devices and diagnostics. Latterly he was the international finance director for Ortho Clinical Diagnostics covering Europe, Africa and Asia. He now works for several start up businesses.

Patrick Brosnan
Consultant
Patrick has a depth of experience in wound care. More recently he has expanded his skills / experience in clinical trial management helping run, monitor, and analyze trials in the USA. Patrick graduated from the University of Manchester with a BSC (hons) in Biology in 2008 and the University of Massachusetts with an MBA in 2022.

Gill Witherington
Administrator
Gill has a long history working in wound care business development, firstly for Johnson & Johnson which then became Systagenix Wound Management. Gill worked in Systagenix Research & Development before being transferred to Woundchek Laboratories in 2013 dealing with a diverse range of business development tasks.

Richard Collette
Commercial
Richard Collette is an experienced Commercial Business Leader in international business development for medical products such as diagnostic imaging equipment, tele-care for chronic wound management, IVDs, and implantable devices covering the past 20 years.
Most recently, he was President of the USA and Canada marketing, sales, distribution, and service operation for one of the leading suppliers of implants that restore hearing to those challenged with profound hearing loss.

Leon Okurowski
Mr. Okurowski is the Vice President of Pear Tree Advisors and the co-founder and Vice-President of the Pear Tree Funds. He is also Vice President and Treasurer for various U.S. Boston entities. Mr. Okurowski has over forty years experience in the finance and investment management business.
Mr. Okurowski obtained his MBA from the Whittemore School of Business at the Univerity of New Hampshire, his MS in taxation from Bentley College, and a BS from the United States Naval Academy.
He has served on the boards of several high-tech companies. He was a co-founder and served on theboard of Atlantic Bank and Trust Company, a public company.
Prior to joining U.S. Boston, Mr. Okurowski served in the United States Navy as a submarine officer.

Bill Umphrey
Mr. Umphrey is the Chairman and co-founder of the Pear Tree Funds, the President of Pear Tree Advisors, Inc., and the Chairman of U.S. Boston Capital Corporation. Mr. Umphrey founded the U.S. Boston's predecessor in 1969 and has over forty years of experience in the finance and investment management business.
Mr. Umphrey obtained his JD from Harvard Law School, an LLM in taxation from Boston University of Law, a Masters in Personnel Administration fron George Washington Univeristy, and a BS with distiction from the United States Naval Academy in Annapolis, Maryland.
Prior to founding U.S. Boston, Mr. Umphrey served in the United States Navy as a submarine officer.

 Events
Events
 News
News
Italian study demonstrates utility of Woundchek’s wound diagnostic tests.
Date: Sep 15, 2022
Professor Claudio Ligresti led a study in four Italian wound clinics where forty chronic ulcers were treated with the therapy decisions assisted by WOUNDCHEK™ Bacterial Status and WOUNDCHEK™ Protease Status tests.
Testing was repeated and outcomes monitored after approximately fifteen and thirty days. The outcomes and treatments employed were compared with a cohort that were not tested. Using the two point of care test results to inform treatment decisions was strongly associated with increased rates of healing and improvement.
Professor Ligresti found that the rapid test results were extremely valuable in selecting treatments and monitoring effectiveness and especially useful in targeted use of advanced therapies and invasive surgical interventions.
The study confirmed that using WOUNDCHEK™ Bacterial Status and WOUNDCHEK™ Protease Status tests can assist treatment decisions and improve healing.
 Contact Us
Contact Us
WOUNDCHEK™ Laboratories
CIE Bld.,
151 Martine St., Fall River,
Massachusetts, 02723,
United States of America
T: 888 685 0060
Airebank Mill
Gargrave; BD23 3RX
North Yorkshire
United Kingdom
T: +44 (0) 1756 639111
E: info@woundchek.com
Technical Helpline:
Americas: 888 685 0060
ROW: +800 1558 8551
 Our Distributors
Our Distributors
For a complete list of our distributors, please submit your request using the form.
If you are interested in becoming a distributor, please submit your request using the form.